Most new antibiotic approvals for food animals have been generic drugs also used for humans
- by Stacy Sneeringer and Dennis Vilorio
- 5/11/2020
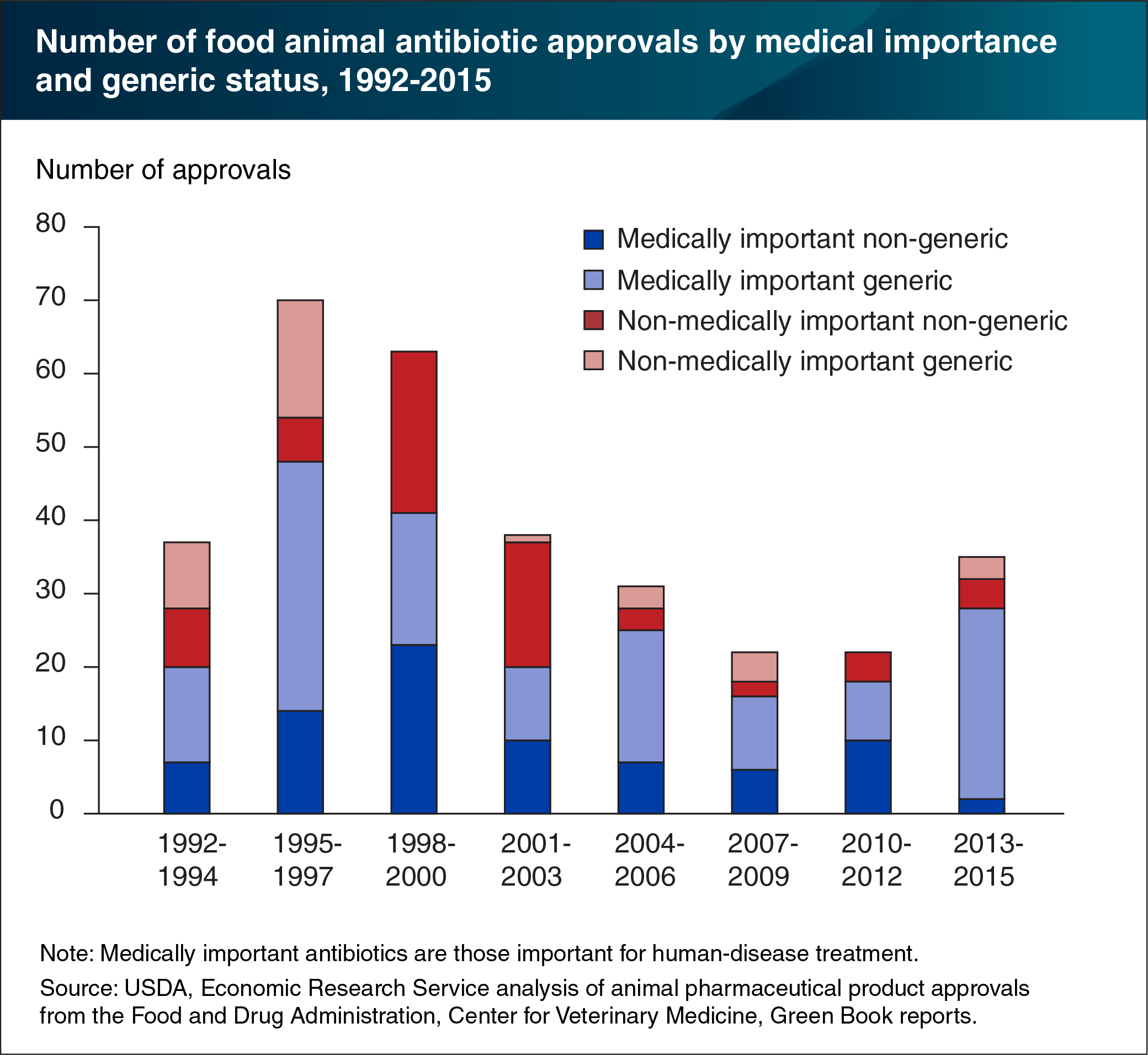
U.S. regulations on antibiotic use in food animal production have focused on antibiotics important for human disease treatment, which the Food and Drug Administration (FDA) terms “medically important.” If current human antibiotics lose efficacy and new ones are not developed, the ability to treat human infections may be hindered. In 2017, FDA policies ended the use of medically important antibiotics for growth promotion in food animals. Antibiotics deemed “currently not medically important” are not used to treat human illnesses and can still be used for animal growth promotion. Other uses of medically important antibiotics in food animal production require veterinarian oversight. These policies follow earlier actions in the European Union (EU) banning medically important antibiotics for growth promotion. Most new antibiotic approvals for food animals have been generic drugs that are also used for humans. Between 1992 and 2015, about 70 percent of antibiotics were considered medically important. This suggests that animal pharmaceutical companies are increasingly developing generic antibiotics for food animals, not new varieties of antibiotics. Although new approvals for non-medically important, non-generic antibiotics for food animals declined, they still averaged about 1.3 per year in 2015. This chart appears in the ERS report, The U.S. and EU Animal Pharmaceutical Industries in the Age of Antibiotic Resistance, released May 2019.

